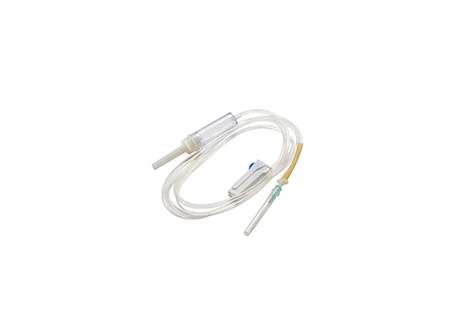
Infusion set A1
Infusion set A1
Soft drip chamber with medicate port
With PVC tube, length 1.5m and extra tube with filter
with normal flow regulator
with luer lock connector
with scalp vein set
Sterilized by EO gas, non-toxic, non-pyrogenic
Instructions for Use
Sterile Infusion Sets for Single Use (with Needle)
- Please read these instructions carefully before using this product.
- Keep these instructions for reference.
- In case of any serious incident please report it to the manufacturer and your competent authority.
- These instructions for use are based on experience from physicians and/or their published literature. It is intended to assist in using this device. It is not a reference to surgical techniques.
1. PRODUCT NAME
Sterile Infusion Sets for Single Use (with Needle)
2. DESCRIPTION/INDICATIONS
Sterile infusion sets for single use (with needle) is a medical device that is used for general purpose of
intravenous infusion of fluids and medications into the human body. It is attached with an intravenous needle of stainless steel.
Two types of infusion set are available: type ZY- 1 and type ZY-2. In comparison to the type ZY-2 the type ZY- 1 include an injection site with tubing for the purpose that additional medications can be injected during the infusion. Through the closure-piercing device, the infusion set will be connected to a container for
Infusion. 20 drops of distilled water delivered by the drip tube are equivalent to a volume of 1ml ± 0.1ml. The fluid filter is positioned near the patient access. It has a nominal pore size of 15μm.
As main component of the infusion set, the tubing is made of PVC, while the needle is made of stainless steel. The DEHP-free PVC type MT(E)-NT is used in the device. In this PVC-type, Tri-2-Ethylexyl
Trimellitate (TOTM) is applied as plasticizer, this substance does not belong to substances of very high concern (SVHC) as listed in REACH Regulation.
The infusion set is provided with protective caps to maintain sterility of the internal parts of the set until the set is used. The air-inlet device is provided with a protective cap over the closure-piercing device and needle. The needle has a nominal outside diameter of 0.4 mm, 0.45 mm, 0.5 mm, 0.55 mm, 0.6 mm, 0.7 mm, 0.8
mm, 0.9 mm, 1.1 mm or 1.2 mm. The nominal length of the intravenous needle ranges from 13 mm to 30
mm. All of them have the same general features. The wall thickness of the needle is divided into regular wall (RW) and thin wall (TW). According to the primary bevel angle, the needles differ in short bevel (SB) and
long bevel (LB).
The sterile infusion set for single use (with needle) is manufactured acc. to EN ISO 8536-4, and the needle is conforming to EN ISO 7864. The intravenous needles are identified with colour coding acc. to EN ISO 6009. The connection between the tubing and needle is friction-fit connection with a 6% (Luer) taper acc. to EN
ISO 80369-7.
The device is delivered in a sterile state with EO sterilization. The shelf life is defined for 3 years after sterilization. A re-sterilization by users is not allowed.
Intended Purpose
Sterile infusion set for single use (with needle) is intended for general purpose of intravenous infusion of fluids and medications into the human body.
It is intended for gravity infusion only.
3. SPECIFICATION
Table 1 Types and Dimensional Specifications of Needle Included in Sterile Infusion Sets (mm)
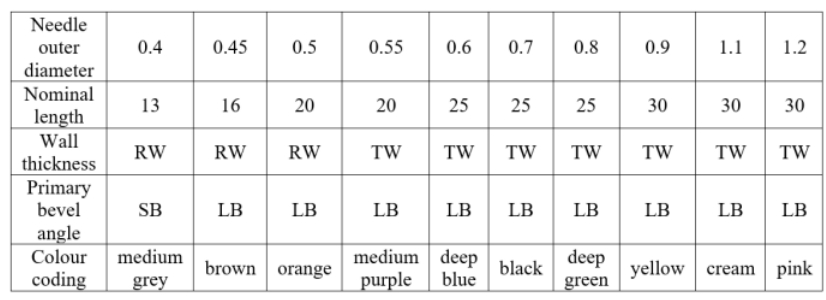
Key: RW (Regular Wall), TW (Thin Wall), SB (Short Bevel), LB (Long Bevel)
4. CONTRAINDICATIONS
The device is contraindicated for use with insulin.
The device is contraindicated for patients who are allergic to PVC.
5. CAUTIONS and WARNINGS
• Carefully read all instructions prior to use. Observe all warnings and cautions. Failure to do so may result in injury.
• The use of this product is restricted to a qualified physician with adequate training and knowledge of these procedures.
• Inspect the integrity of the packaging before use. Do not use if package is open or damaged.
• Upon removal from the package, inspect the product to ensure no damage has occurred.
• The device is intended for gravity infusion only.
• This device should not store and infuse fat-soluble liquids and drugs such as fat emulsion.
• This device should not be used for infusion of drugs that are incompatible with polyvinyl chloride (PVC).
• After use this device maybe a potential biohazard. Handle and dispose of in accordance with acceptable medical practices and with applicable local, state and federal laws and regulations.
• Sterile infusion sets for single use (with needle) are provided in sterile state and intended for use in a single procedure only. Do notre-use, reprocess or resterilize. An infection or transmission of diseases could occur,if the device were to be re-used.
6. POTENTIAL COMPLICATIONS
The act of piercing the skin with a needle, while necessary for an infusion, may cause localized pain.
A needlestick infection can occur when pathogens are inadvertently introduced into the tissues of the body
during an infusion. Contamination of the needle used for infusion, or reuse of needles for infusion in multiple people, can lead to transmission of bloodstream infections.
7. DIRECTIONS FOR USE
(1) Open the sterile package, take out the infusion set by use aseptic method.
(2) Insert the puncture device into the infusion bottle.
(3) Open the flow regulator to evacuate the air from the tubing system.
(4) Prick the infusion needle into the vein. During venipuncture (the insertion of a needle into avein), hold the butterfly needle by its wings between the thumb and index finger.
(5) The short, thin needle is inserted toward avein at a shallow angle. Once inserted, the venous pressure will force a small amount of blood into the transparent tubing, providing confirmation that the needle is
correctly placed.
(6) The wings can also serve to stabilize the needle once it is in place, preventing it from rolling or shifting.
(7) Once used, the entire unit is thrown away in a sharps disposal container. The puncture wound is then bandaged.
8. STORAGE
Keep away from sunlight.
Temperature: < 54 °C
Relative Humidity: 0% to 80%
9. SHELF LIFE 3 years.
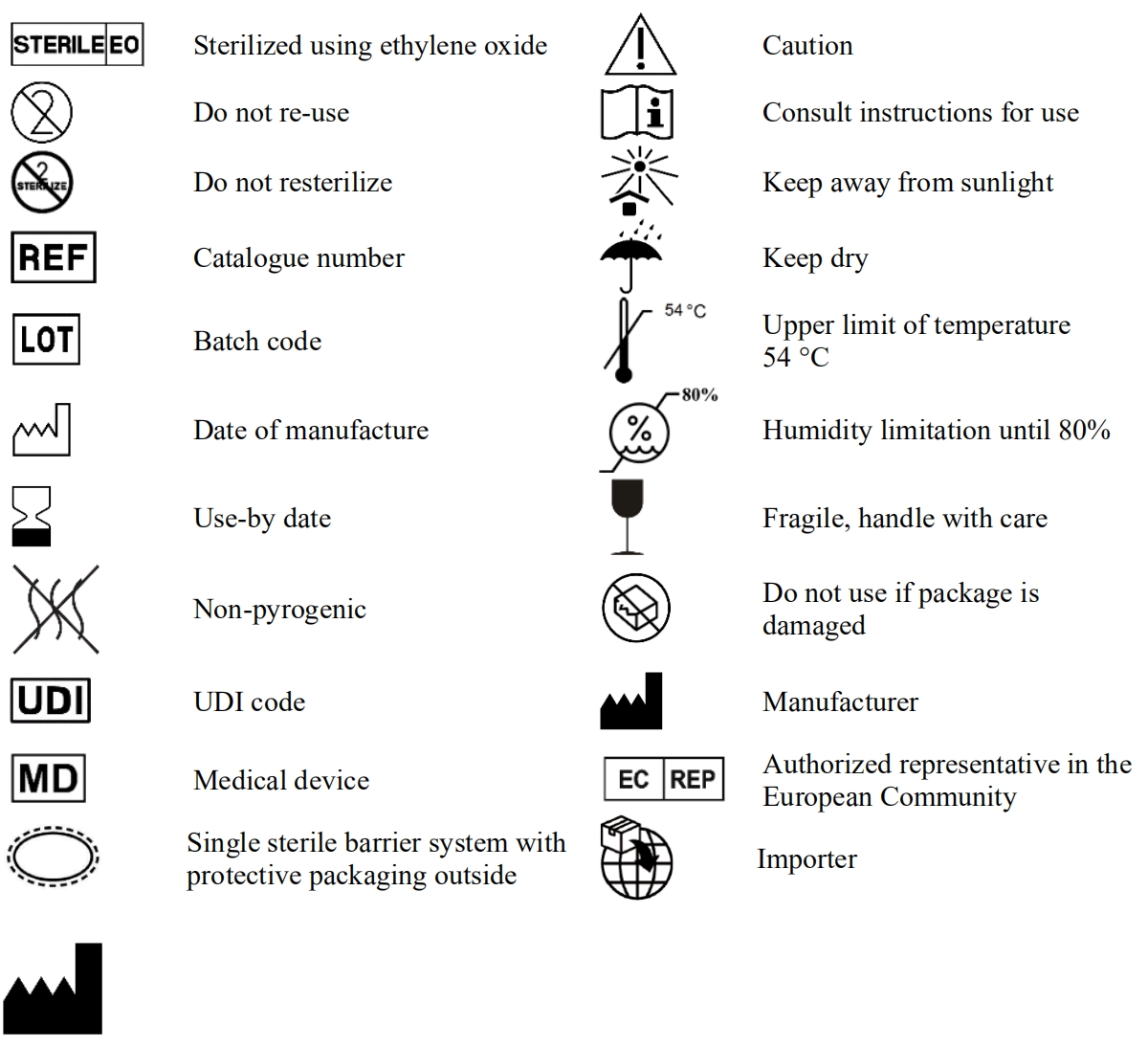
10. EXPLANATION OF SYMBOLS USED ON LABELS AND INSTRUCTIONS FOR USE:
Jiangsu Zhiyu Medical Instrument Co., Ltd.
No. 88 NanYuan Road, Industrial Park, West TaiXing City, 254000 Jiangsu, P.R. China

Prolinx GmbH Brehmstr. 56, 40239 Duesseldorf, Germany
Date of issue: 2022-02- 15 Revision: A/0